Unpacking the Science Why Hastelloy C276 Bars Defeat Pitting
A Hastelloy C276 bar demonstrates remarkable resistance to pitting corrosion. This superior performance comes directly from its high concentrations of Molybdenum and Chromium. These elements synergize to create a highly stable passive film on the alloy's surface.
This protective layer rapidly self-heals when damaged. It provides a robust and reliable defense against localized attacks in aggressive environments, ensuring structural integrity where failure is not an option.
Key Takeaways
- Hastelloy C276 bars resist pitting corrosion very well. This is because they have a lot of Molybdenum and Chromium.
- Molybdenum is the main element that stops pitting. It forms a strong shield against harmful chemicals like chlorides.
- Chromium helps create a protective layer on the metal. This layer can fix itself if it gets damaged.
- Hastelloy C276 has very little carbon. This means it stays strong and resists corrosion even after welding.
- The Pitting Resistance Equivalent Number (PREN) shows how good a metal is at fighting pits. Hastelloy C276 has a very high PREN score.
Understanding Pitting Corrosion: The Silent Threat

Pitting corrosion represents a localized and insidious form of material degradation. Unlike uniform corrosion that evenly reduces a material's thickness, pitting attacks a metal at specific points. This process creates small cavities, or "pits," that penetrate the material, often leaving the surrounding surface unaffected.
What is Pitting Corrosion?
The pitting process begins when a metal's protective passive film breaks down in a localized area. Several factors can trigger this initial breach:
- Aggressive Anions: Aggressive ions, especially chlorides, attack and dissolve the passive layer.
- Surface Imperfections: Microscopic scratches, impurities, or defects on the metal surface create vulnerable points.
- Mechanical Damage: Physical impacts or abrasions can compromise the protective film, exposing the underlying metal to the corrosive environment.
Once initiated, the pit becomes an active corrosion site that can grow rapidly, burrowing deep into the component.
How Pitting Leads to Catastrophic Failure
The primary danger of pitting corrosion is its ability to cause sudden, unexpected structural failure. A component may appear structurally sound on the surface while extensive damage hides beneath. Pits act as stress concentrators, significantly reducing a material's load-bearing capacity and fatigue life. This hidden progression can lead to catastrophic results, as seen in the 2020 shutdown of the FPSO Sevan Hummingbird due to accelerated pitting in its cargo tanks.
The growth of a corrosion pit is an autocatalytic process. This means the corrosive environment inside the pit accelerates its own expansion, allowing it to propagate quickly and deeply into the metal.
This rapid, localized material loss undermines the component's integrity, creating a high risk of failure without obvious external warning signs.
The Role of Chloride-Rich Environments
Chloride ions (Cl-) are the primary catalyst for pitting corrosion in many alloys. These aggressive ions possess a unique ability to dismantle the passive film that normally protects a metal. Chlorides adsorb onto the surface, penetrate the film through weak points, and form soluble metal compounds. This action effectively dissolves the protective layer from within, allowing corrosion to begin.
Common industrial and natural environments present a high risk due to significant chloride concentrations: 🧪
- Seawater and marine atmospheres
- Industrial brines used in chemical processing
- Soils with high salt content
- Roadways and infrastructure exposed to deicing salts
In these settings, standard alloys become highly susceptible to rapid pitting attacks, making specialized materials essential for reliability.
The Chemical Shield of a Hastelloy C276 Bar
The exceptional performance of a Hastelloy C276 bar originates from its meticulously engineered chemical composition. This alloy is not merely a mixture of metals; it is a sophisticated system where each element performs a specific, synergistic function. The high concentrations of Molybdenum, Chromium, Nickel, and Tungsten work in concert to create a formidable defense against pitting and other forms of corrosion.
Molybdenum: The Primary Defense Against Pitting
Molybdenum is the most critical element in Hastelloy C276 for combating pitting corrosion, especially in chloride-rich environments. Its defensive actions are multifaceted and highly effective. Molybdenum significantly enhances the stability of the passive film, acting as a powerful deterrent to localized attacks.
The protective mechanisms of Molybdenum are complex and operate at multiple levels:
- It forms robust Molybdenum oxide species within the passive layer, creating a physical barrier that impedes the penetration of chloride ions.
- It strategically positions itself at defect sites within the protective film, preventing chlorides from breaching the primary Chromium oxide barrier.
- It actively "cures" weak points in the alloy's surface by promoting the replacement of less-resistant elements, like iron, with more stable Chromium and Molybdenum compounds.
- It stabilizes the oxide matrix by discouraging the formation of oxygen vacancies, which are entry points for aggressive chlorides.
Molybdenum and Tungsten do not rely on a single mechanism. Instead, they employ various strategies at different stages of the corrosion process to bolster the alloy's resistance.
This dynamic and multi-layered defense makes Molybdenum the alloy's frontline soldier against pitting.
Chromium: The Foundation of the Passive Layer
Chromium provides the fundamental framework for corrosion resistance in many alloys, including Hastelloy C276. It reacts with oxygen to form a thin, tenacious, and self-healing layer of chromium oxide (Cr₂O₃) on the metal's surface. This "passive film" is the initial barrier that separates the underlying alloy from the corrosive environment.
While the chromium content of Hastelloy C276 is similar to that of some standard stainless steels, its effectiveness is amplified by the other elements in the alloy.
| Material | Chromium Content |
|---|---|
| Hastelloy C276 | ~16% |
| 316L Stainless Steel | 16-18% |
In Hastelloy C276, the chromium-based passive film is powerfully reinforced by Molybdenum, making it far more resilient to chloride-induced breakdown than the film on standard stainless steels.
Nickel and Tungsten: The Supporting Elements
Nickel and Tungsten are essential supporting elements that provide the strength, stability, and toughness required for high-performance applications.
Nickel, as the primary constituent of the alloy, forms the matrix that holds all other elements together. It imparts exceptional ductility and toughness, allowing the Hastelloy C276 bar to be fabricated and to withstand mechanical stress without fracturing. Nickel is a key component that provides overall strength to the alloy, ensuring it maintains its structural integrity even in harsh conditions.
Tungsten further enhances the alloy's capabilities, particularly in extreme environments. Its contributions are significant:
- Enhanced Strength: Tungsten provides a major boost to the alloy's strength, especially at elevated temperatures. This solid solution strengthening improves resistance to deformation and creep.
- Improved Corrosion Resistance: It works alongside Molybdenum to increase resistance to pitting and crevice corrosion.
- Increased Chemical Stability: Tungsten helps the alloy resist stress corrosion cracking, making it reliable in aggressive chemical processing environments.
Together, Nickel and Tungsten create a robust and resilient foundation, allowing the primary defensive elements, Molybdenum and Chromium, to perform their functions with maximum effectiveness.
Quantifying the Defense: How C276 Stops Pitting
The superior corrosion resistance of Hastelloy C276 is not just an observation; it is a measurable and predictable property. Engineers and metallurgists use specific metrics and an understanding of electrochemical processes to quantify this defense. The Pitting Resistance Equivalent Number (PREN) provides a numerical rating, while the mechanism of rapid repassivation explains how the alloy actively heals itself to stop corrosion in its tracks.
The Pitting Resistance Equivalent Number (PREN)
The Pitting Resistance Equivalent Number (PREN) is a calculated value that helps predict an alloy's resistance to localized pitting corrosion. This number provides a standardized way to compare different materials. A higher PREN indicates superior resistance. The calculation weighs the contributions of key elements, with Molybdenum and Tungsten receiving significant emphasis.
Several formulas exist to determine PREN, but they all highlight the importance of Chromium, Molybdenum, Tungsten, and Nitrogen.
PREN = %Cr + 3.3(%Mo) + 16(%N)PREN = %Cr + 3.3 x (%Mo + 0.5 x %W) + 16 x %N
The second formula is particularly relevant for tungsten-bearing alloys like Hastelloy C276. It shows how Tungsten works with Molybdenum to boost pitting resistance. When compared to other high-performance alloys, the PREN of Hastelloy C276Bars demonstrates its elite status. For many seawater applications, a PREN above 40 is considered necessary. Hastelloy C276 far exceeds this benchmark.
| Alloy | Typical PREN Value |
|---|---|
| 316L Stainless Steel | ~25 |
| Super Duplex Stainless Steel | >40 |
| Alloy 625 | ~46-56 |
| Hastelloy C276 | ~64-73 |
This high PREN value quantifies the powerful chemical shield created by the alloy's composition, making a Hastelloy C276 bar a scientifically validated choice for critical service.
The Mechanism of Rapid Repassivation
While PREN is an excellent predictive tool, the true defensive power of Hastelloy C276 lies in its dynamic ability to self-heal. This process, known as repassivation, is the rapid reformation of the protective passive film after it has been mechanically or chemically damaged. This healing capability is what truly stops a potential pit from growing into a catastrophic failure.
The repassivation process is a swift, multi-stage electrochemical response:
- Breach and Exposure: An aggressive chloride ion or a microscopic scratch breaches the chromium-oxide passive layer, exposing the raw alloy beneath.
- Molybdenum's Response: Molybdenum immediately goes to work. It stabilizes the area around the breach, preventing rapid metal dissolution. It forms highly stable Molybdenum oxides that "plug" the hole and make the local environment less corrosive. 3An Chromium's Reinforcement: The initial breach creates vacancies in the alloy's surface. This encourages Chromium atoms to diffuse outward to the surface, where they react with oxygen to rebuild the primary chromium-oxide passive film.
- Permanent Seal: The combined action of Molybdenum and Chromium quickly seals the defect. The reformed passive layer is often stronger and more resistant than the original film, effectively neutralizing the threat.
This ability to rapidly repair damage is the essence of the alloy's resilience. It does not just resist attack; it actively fights back on a microscopic level to maintain its integrity.
This rapid repassivation mechanism ensures that minor surface imperfections or incidental damage do not escalate into dangerous pitting, providing unmatched reliability in the most demanding environments.
The Low-Carbon Advantage in Welded Applications
The process of welding subjects a metal to intense, localized heat. This thermal cycle can compromise the corrosion resistance of many alloys, creating weak points prone to failure. Hastelloy C276, however, is engineered with a specific chemical advantage to overcome this very problem. Its extremely low carbon content ensures that its superior performance remains intact even after fabrication.
Preventing Sensitization and Carbide Formation
Welding can trigger a damaging phenomenon in many alloys known as sensitization. During the welding process, areas of the metal are heated to a critical temperature range, typically between 800°F and 1600°F (427°C and 816°C). In alloys with higher carbon content, this heat causes carbon to react with chromium, forming chromium carbide precipitates along the material's grain boundaries. This process depletes the chromium needed to maintain the protective passive film, creating pathways for corrosion.
Hastelloy C276 effectively eliminates this risk through its carefully controlled chemistry. The alloy contains a maximum carbon content of just 0.01%, as specified by standards like ASTM B574.
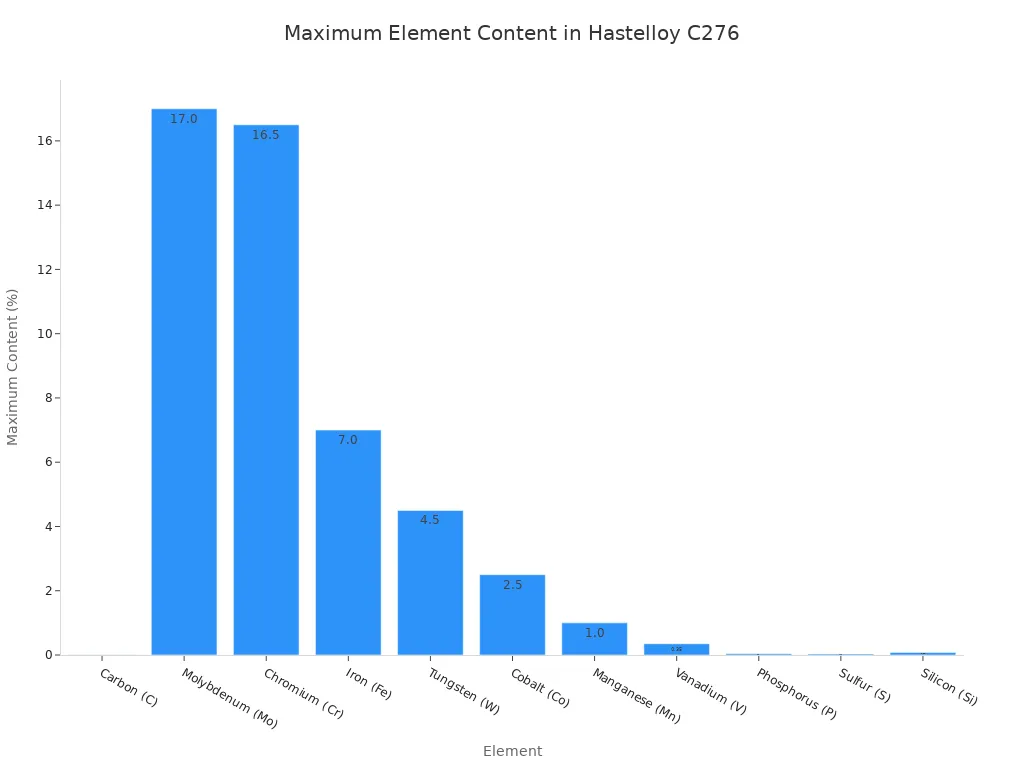
With virtually no carbon available to form carbides, the chromium remains dissolved within the alloy matrix, ready to perform its protective function.
Maintaining Corrosion Resistance in Heat-Affected Zones
The area directly adjacent to a weld is called the Heat-Affected Zone (HAZ). This zone does not melt but experiences significant thermal stress, making it the primary location for sensitization and subsequent weld decay in susceptible materials.
The low-carbon design of Hastelloy C276 directly protects the HAZ. By preventing the formation of grain-boundary carbides, the alloy ensures that the corrosion resistance of the HAZ is identical to that of the base metal. The chromium and molybdenum remain fully effective, preserving the integrity of the passive layer throughout the entire component.
This exceptional thermal stability allows Hastelloy C276 to be used in the "as-welded" condition for most chemical process applications. It eliminates the need for costly and time-consuming post-weld heat treatments to redissolve precipitated carbides.
This makes a Hastelloy C276 bar a reliable and practical solution for fabricated equipment destined for the most aggressive service environments. 🏭
The scientific design of a Hastelloy C276 bar provides its elite defense against pitting. A powerful synergy between high Molybdenum and Chromium content, measured by a superior Pitting Resistance Equivalent Number (PREN), creates the core shield. This primary defense is reinforced by a low-carbon, high-nickel matrix that maintains corrosion resistance in welded components.
This precise metallurgical engineering makes the Hastelloy C276 bar an unparalleled and reliable solution for the most aggressive environments, from chemical processing equipment to marine pipelines, where failure is not an option. 🛡️
FAQ
What makes Molybdenum and Chromium so important in Hastelloy C276?
Chromium creates the primary protective passive film on the alloy's surface. Molybdenum significantly strengthens this film. It prevents chloride ions from causing localized pitting corrosion, ensuring a superior defense against attacks.
Can Hastelloy C276 bars be used in high-temperature environments?
Yes. Hastelloy C276 maintains excellent mechanical properties at elevated temperatures. This characteristic makes the alloy a reliable choice for demanding high-temperature applications where both strength and corrosion resistance are necessary. 🔥
How does Hastelloy C276 compare to standard stainless steel?
Hastelloy C276 offers vastly superior pitting resistance. Its high Molybdenum content results in a much higher Pitting Resistance Equivalent Number (PREN). This makes it suitable for aggressive environments where standard stainless steels would quickly fail.
Why is low carbon content crucial for welded Hastelloy C276?
The low carbon content prevents the formation of harmful chromium carbides during welding. This process, known as sensitization, depletes corrosion resistance. C276's chemistry preserves the integrity of its heat-affected zones, eliminating the need for post-weld heat treatment.
See Also
Mastering Pass Partition Plate Welding: Expert Strategies for 2025 Success
Optimizing Steel Processing for Heat Exchangers: Consistent Performance and Results
Unveiling Molybdenum High-Temperature Furnaces: Design, Function, and Innovation
Molybdenum High-Temperature Furnaces: A Comprehensive Guide for 2025 Insights
Assessing Molybdenum Plugs: Enhancing Seamless Steel Pipe Production by 2025